From the Resene One-Line Specification Manual
The meaning of the word corrosion is "the deterioration of the substance (usually a metal) or its properties because of a reaction with its environment". Normally it specifically applies to metals, although plastics and other non-metals such as concrete, bricks and timber also deteriorate in natural environments. Corrosion causes enormous losses, which rise yearly with the increased usage of metals in industrial development. The accepted concept of corrosion is that it is a result of an electrochemical reaction taking place on the surface of the metal where the metal is converted into metal oxides or other corrosion products.
With some metals, they produce a tight skin on the metal surface, which hinders further corrosion, and if this surface layer is broken it is self-healing. These metals are said to be passivated and include lead, nickel, cadmium, chromium and aluminium. Zinc corrosion products form a fairly tight layer on zinc and further corrosion is slow. A tight layer of iron and chromium oxides forms on the surface of stainless steel and is the reason for the resistance of this metal. Iron and steel, however, form rust as a corrosion product, which is porous, is not firmly adherent and does not prevent continued corrosion.
Steel is an alloy or mixture of iron and up to 1.7% carbon, sometimes with small quantities of elements such as manganese, phosphorous, sulphur and silicon. The corrosion resistance of steel is dominated by interactions between the constituents of the steel. The steel surface contains both anodic and cathodic sites. In the presence of a surface layer of water or other conducting solution an electric current passes between the anodes and cathodes. By convention the transmission of the current is by electrons, which are the electrical charges attached to atoms, and are generated at anodes. Their loss leaves the anodic areas deficient in electrons and iron goes into solution as ferrous ions viz.

This reaction is the basis of the corrosion of iron. Electrons generated as shown above are consumed at the cathode area, and react there in various ways depending on the availability of oxygen. In normal atmospheric corrosion there is an ample supply of oxygen and the following reaction occurs.

The hydroxyl ions (OH-) from the cathode combine with the ferrous ions (Fe+2) from the anode to form ferrous hydroxide, which is precipitated. This further reacts with oxygen and water to form hydrated ferric oxide, which is known as rust. This is shown as:
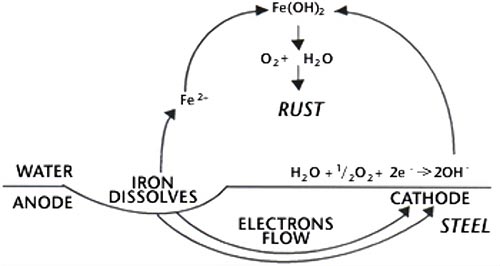
The rate of rust formation rapidly increases if the transfer of electrons from the anode to the cathode can be made easier; which happens if the conductivity of the water increases. This can occur due to the presence of dissolved salts, such as sea salts, on the surface or sulphur products from pollution fallout.
In atmospheric corrosion it has been found that moisture is the controlling factor in the rate of rust formation and little rusting occurs unless the relative humidity is above 60-70%.
In contact with acids corrosion increases because of direct attack on the metal, while under alkaline conditions the rusting of iron is inhibited.
Three methods may be used:
Anodic end – greater tendency to corrode
Cathodic end – lesser tendency to corrode
(A) = Active metal surfaces
(P) = Passive metal surfaces
Resene One-Line Specifications and Data Sheets
Resene offers clear and simple paint specification guidelines, designed to make specifying the right paint system easier. If in doubt about any aspect of your specification please contact Resene.